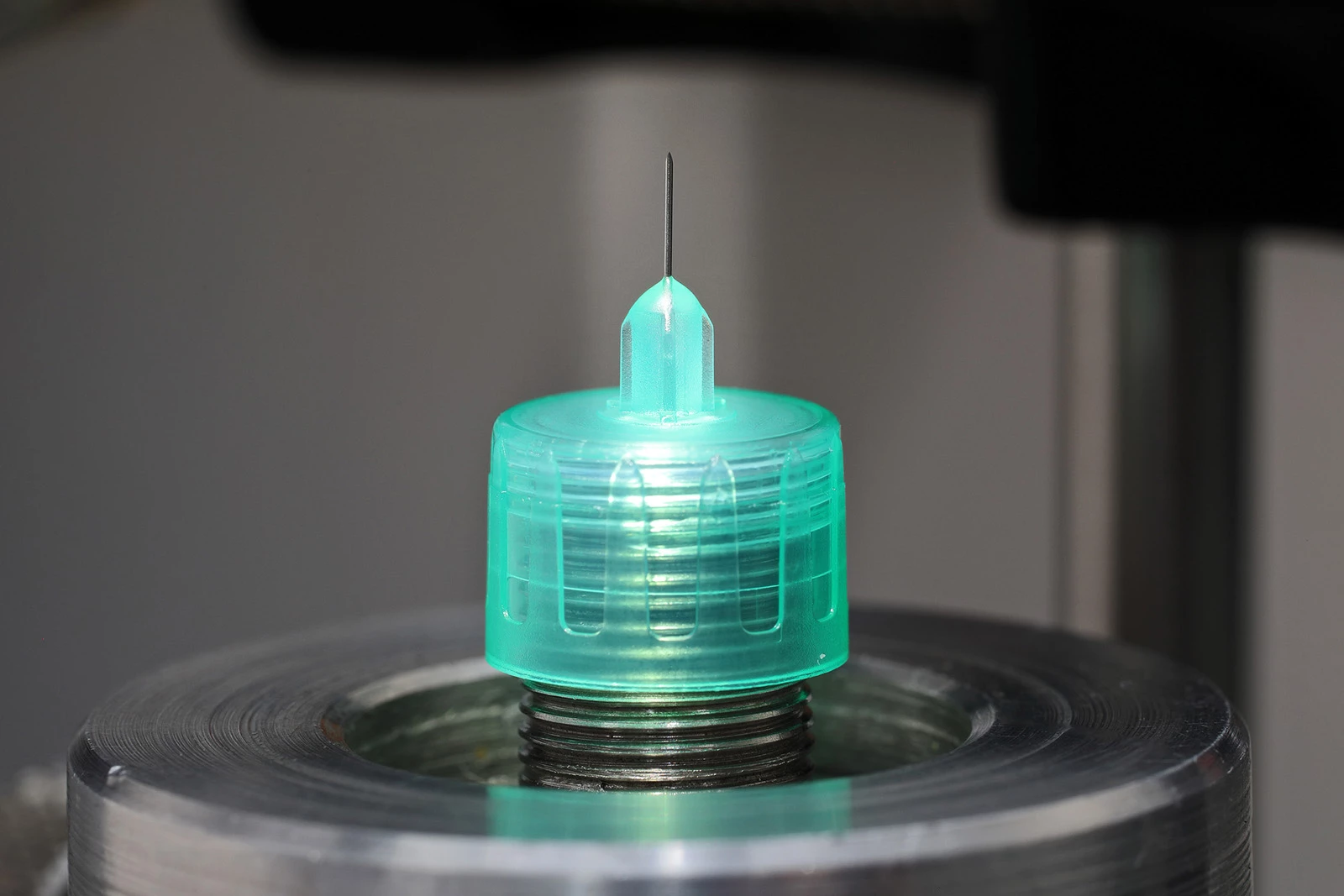
Pre-filled syringes are convenient for self administration, carers, emergency situations as well as more general use. The range of treatments to be found is large and growing. Just considering the letter ‘A’, we have: anti-thrombosis (Enoxaparin), arthritis treatment (Abatacept), and antiseptic (dental hypochlorite). Every new syringe/formulation combination requires full performance and safety validation for FDA submission and EC registration. The key parameters to analyse are: Delivered dose, extractables and leachables, toxicity, ISO 11040 performance, stability, and maintenance of sterility. If present, anti-needle stick devices must also be shown to be effective.
MET has the leading programme for comprehensive design validation testing, including all aspects of the relevant ISO syringe standards plus usability studies and measurement of bio-availability. All work is carried out efficiently to agreed protocols, delivering your results in the shortest possible timescale.
Syringe Testing standards include:
- ISO 11040 Pre-filled Syringes
- ISO 80369 Small-bore Connectors for Liquids and Gases in Healthcare Applications
- ISO 23908 Sharps Injury Protection
- ISO 11608-1 Needle-based Injection Systems for Medical Use - Requirements and Test Methods
- ASTM D4169 Standard Practice for Performance Testing of Shipping Containers and Systems
- ISO 7886 Sterile Hypodermic Syringes for Single Use
- ISO 8537 Sterile Single-use Syringes, with or without Needle, for Insulin
- ISO 10993 Biological Evaluation of Medical Devices
- ISO 11607 Packaging for Terminally Sterilized Medical Devices
- ASTM D6653 Standard Methods for Determining the Effects of High Altitude on Packaging Systems by Vacuum Method
- USP 1207 Sterile Product Packaging-Integrity Evaluation
The ISO 11040 series of standards covers all aspects and applications of syringes, there are eight individual parts to the ISO 11040 series, these are:
- ISO 11040-1:2015 - Prefilled syringes - Part 1: Glass Cylinders for Dental Local Anaesthetic Cartridges: This part of the series specifies the materials, design, dimensions and test methods for single-use glass cylinders for dental local anaesthetic cartridges.
- ISO 11040-2:2011 - Prefilled syringes - Part 2: Plunger Stoppers for Dental Local Anaesthetic Cartridges: This part focuses on the specifications (materials, dimensions, performance requirements and labelling) for plunger stoppers used in single-use dental local anaesthetic cartridges.
- ISO 11040-3:2012 - Prefilled syringes - Part 3: Seals for Dental Local Anaesthetic Cartridges: This part focuses on the material, dimensional, performance and labelling requirements of single-use seals for dental local anaesthetic cartridges.
- ISO 11040-4:2024 - Prefilled syringes - Part 4: Glass Barrels for Injectables and Sterilised Subassembled Syringes Ready for Filling: This part specifically addresses the requirements around materials, dimensions and testing for glass barrels for injection preparations and sterilised subassemblies ready for filling.
- ISO 11040-5:2012 - Prefilled syringes - Part 5: Plunger Stoppers for Injectables: This part of the series covers the specifications of plunger stoppers for glass barrels.
- ISO 11040-6:2019 - Prefilled syringes - Part 6: Plastic Barrels for Injectables and Sterilised Subassembled Syringes Ready for Filling: This part provides guidance materials, dimensions and performance requirements for single-use polymer barrels and sterilised subassembled syringes.
- ISO 11040-7:2024 - Prefilled syringes - Part 7: Packaging Systems for Sterilised Subassembled Syringes Ready for Filling: This part specifies the packaging system used to deliver syringes ready for filling in tubs and nests.
- ISO 11040-8:2016 - Prefilled syringes - Part 8: Requirements and Test Methods for Finished Prefilled Syringes: This part provides functional and safety requirements for single-use terminally sterilised pre-filled syringes.
MET has published papers on extractables and leachables testing for pre-filled syringes and PFS design validation, Click here to access our paper on design validation testing for PFSs, or contact us to request your copies.
MET also worked with Cormica to present a webinar. Understanding Critical Components of Pre-Filled Syringes.
Read more about extractables and leachables for drug delivery systems here.
Our laboratories have also developed numerous technologies for characterising PFSs, including an atmospherically controlled, non-contact dimensional measurement system for simulating air transport, and measuring air bubble expansion.