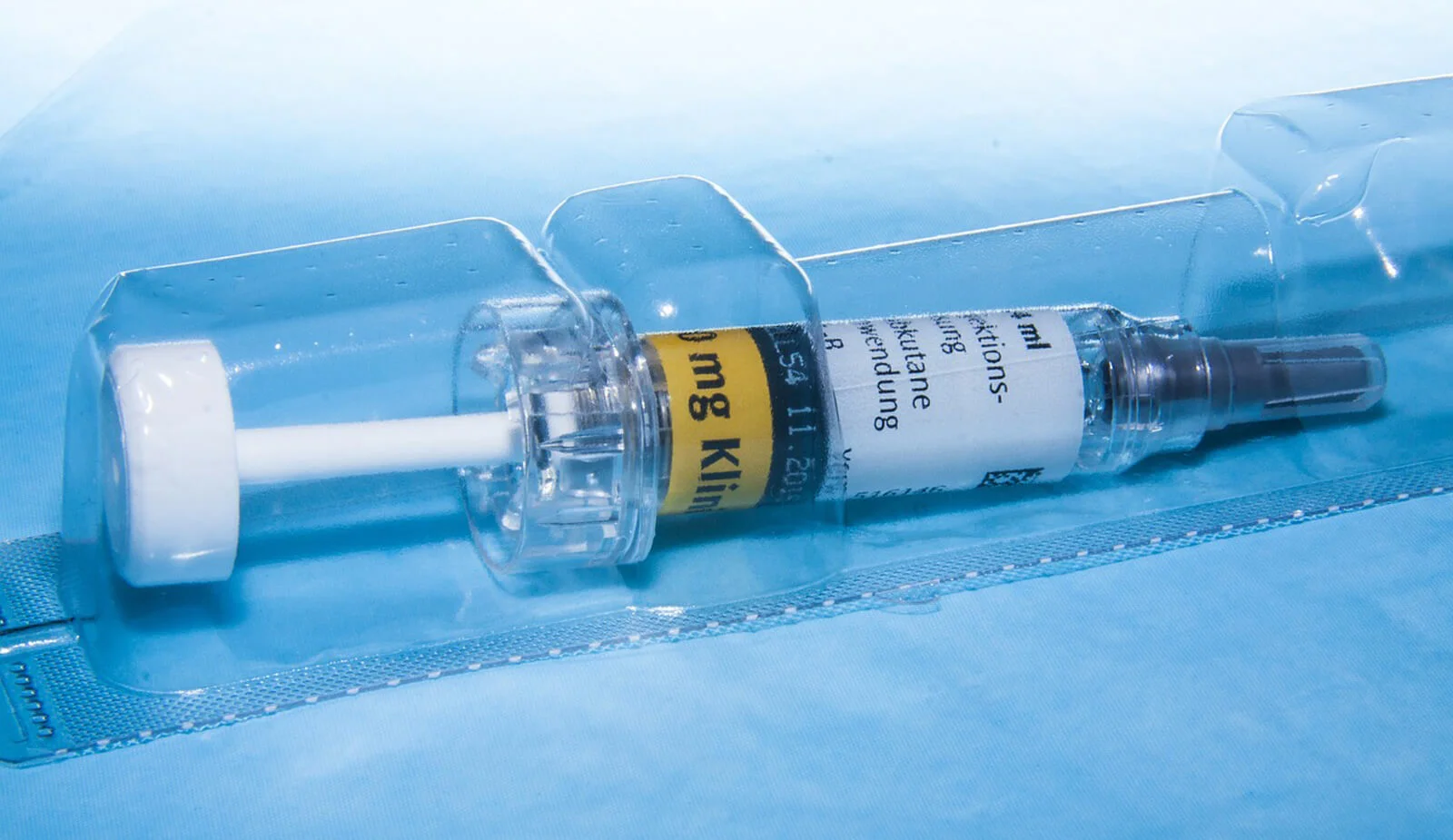
Packaging forms an integral and very important part of all sterile medical devices. CE technical files and FDA submissions both require testing to ISO 11607-1 - Packaging for terminally sterilized medical devices. This is why medical device packaging testing is so important.
MET offer comprehensive medical device packaging testing, to support engineers with the validation of medical pouches and blisters and shelf-life claims for all classes of devices. Our facilities include an ISO/IEC 17025 accredited laboratory, dedicated accelerated ageing ovens and sophisticated burst, leak and tensile testing equipment. We also undertake transit simulation studies, to assess the suitability of shipping cartons or single boxes to protect the products within. Find out more about the kinds of medical device packaging testing we offer below...
Accelerated Ageing
Accelerated ageing is used to simulate real shelf-life ageing and is therefore conducted to validate shelf-life claims. It is carried out according to
ASTM F1980 Standard Guide for Accelerated Ageing of Sterile Medical Device Packages. Read more about Accelerated Ageing. Read more about Accelerated Ageing...
Seal Strength & Integrity
All sterile medical devices require validation of their packaging. The sterile barrier must be shown to be effective throughout the product's claimed shelf-life. Examination of materials, processes and stability all contribute to demonstrating that the sterilisation process is effective and that sterility is maintained. Read more about Seal Strength and Integrity...
Transit Simulation
Shipping validation is mandatory for CE marked medical devices. MET's testing protocols follow the philosophy of ISO 11607-1 - Packaging for terminally sterilized medical devices. Requirements for materials, sterile barrier systems and packaging systems. Read more about Transit Simulation…